TRUSTED BY
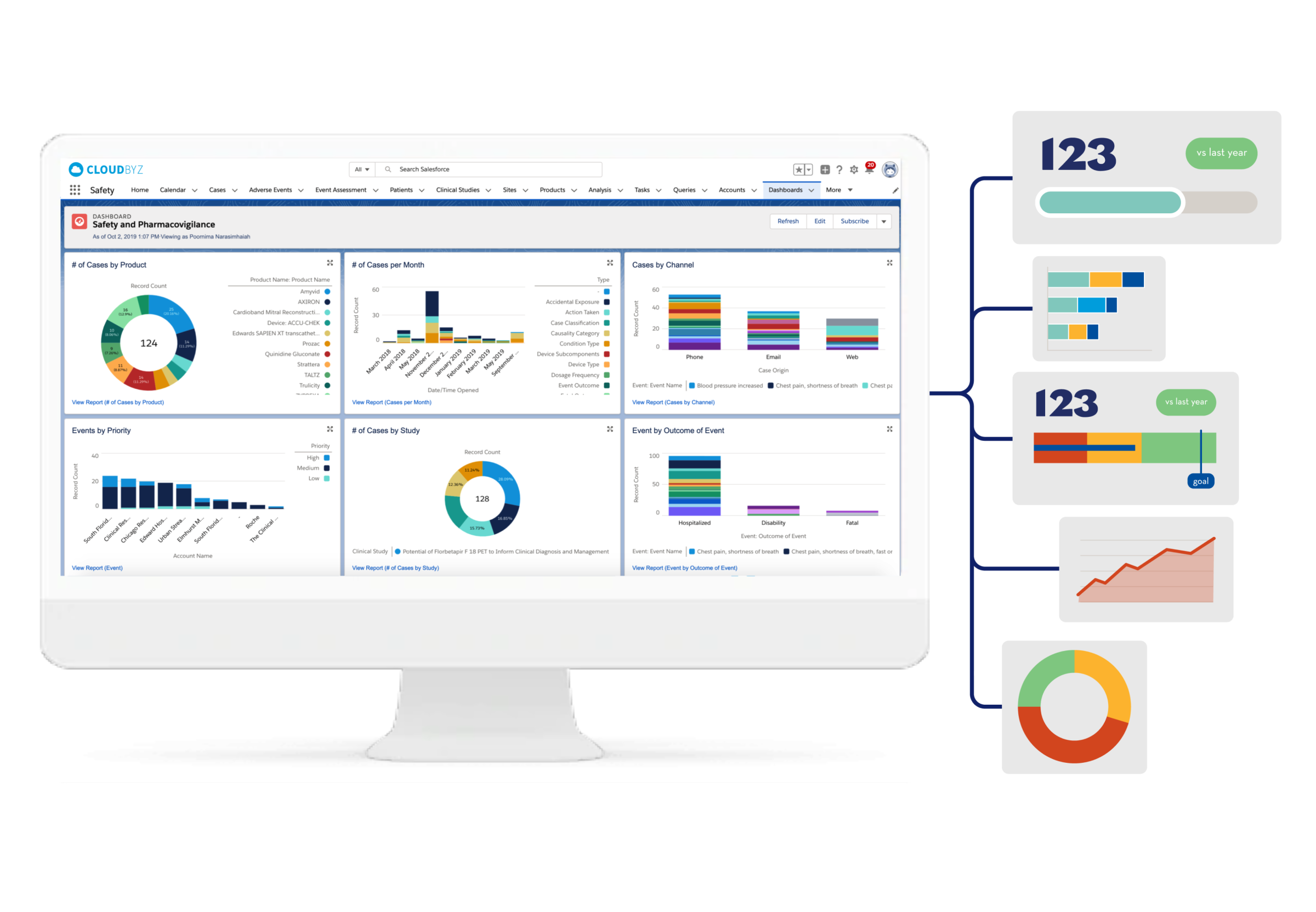
Safety & Pharmacovigilance
Cloudbyz Safety and Pharmacovigilance (PV) database is a cloud-based solution built natively on the Salesforce platform, offering 360 degree view across R&D and commercial. It enables pharma, bio-tech and medical device companies to make faster and better safety decisions by optimizing global pharmacovigilance compliance along with easy to integrate regulatory compliance features.
Our pharmacovigilance database solution easily integrates the required data over a centralized cloud-based platform for advanced analytics set-up along with data integrity. It empowers the end-user with proactive pharmacovigilance, smart features with data-backed predictability, scalability, and cost-effective support.
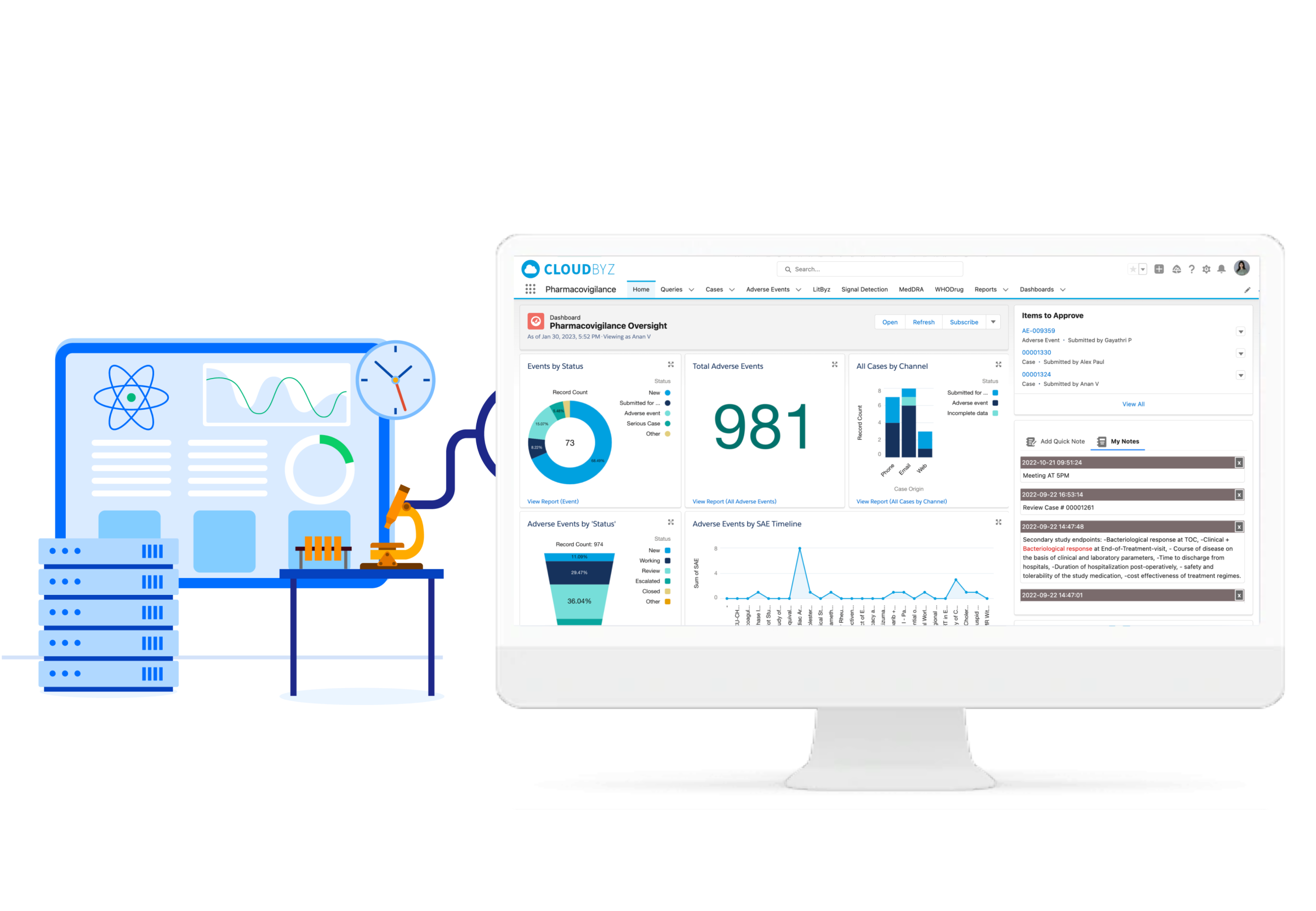
Welcome to Cloudbyz, your trusted partner for intelligent and comprehensive Pharmacovigilance solutions. Our platform ensures enhanced patient safety and streamlined drug surveillance operations.
Cloudbyz recognizes the integral role of pharmacovigilance in maintaining drug safety and regulatory compliance in the healthcare industry. We've designed a cutting-edge solution that tackles the unique challenges of this domain, simplifying processes, enhancing efficiency, and offering reliable, actionable insights.
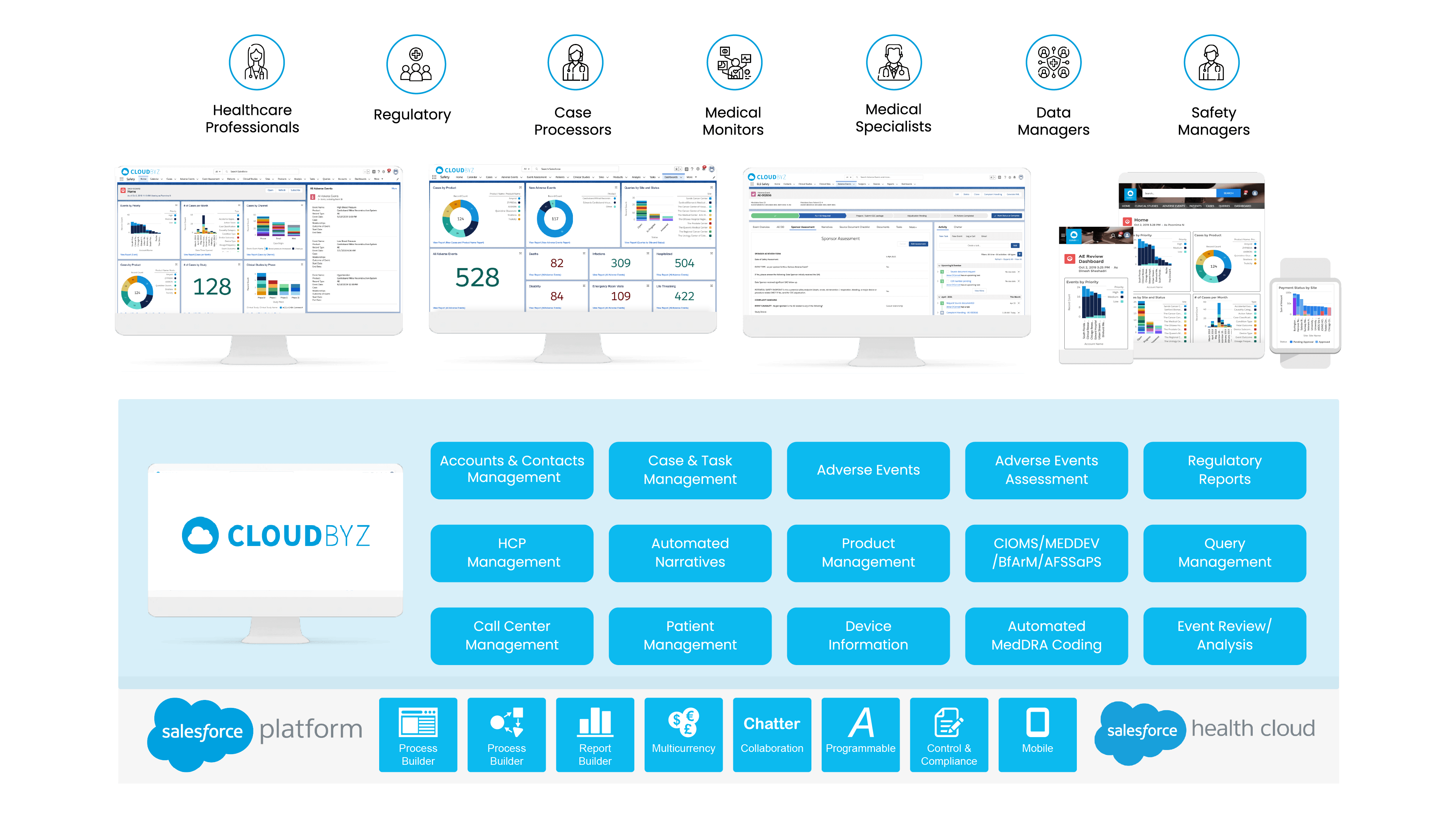
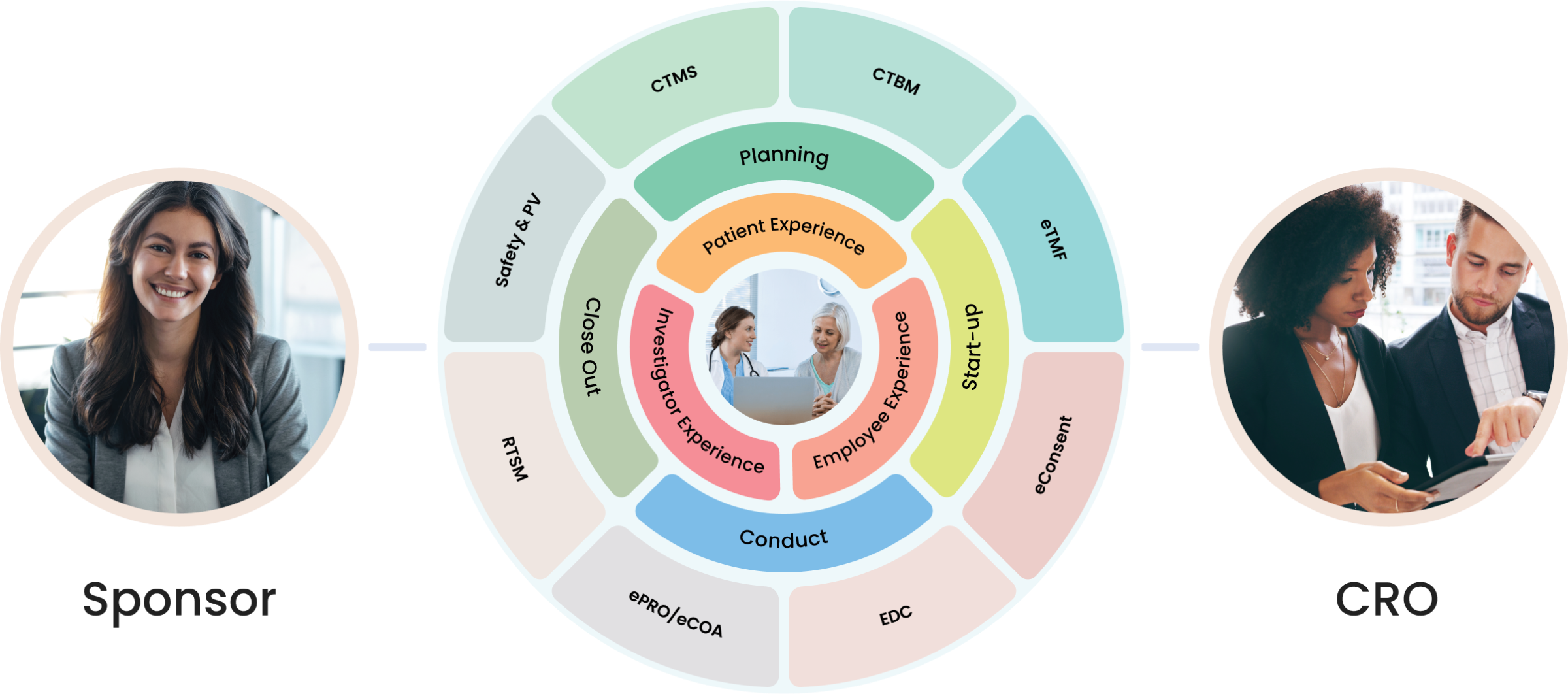
Our pharmacovigilance solution, built natively on the Salesforce platform, allows for proficient collection, analysis, monitoring, and prevention of adverse drug effects. It unifies data from numerous sources, creating a comprehensive hub for managing all your drug safety needs.
Key Features
Automated Case Intake
Automated Case Intake
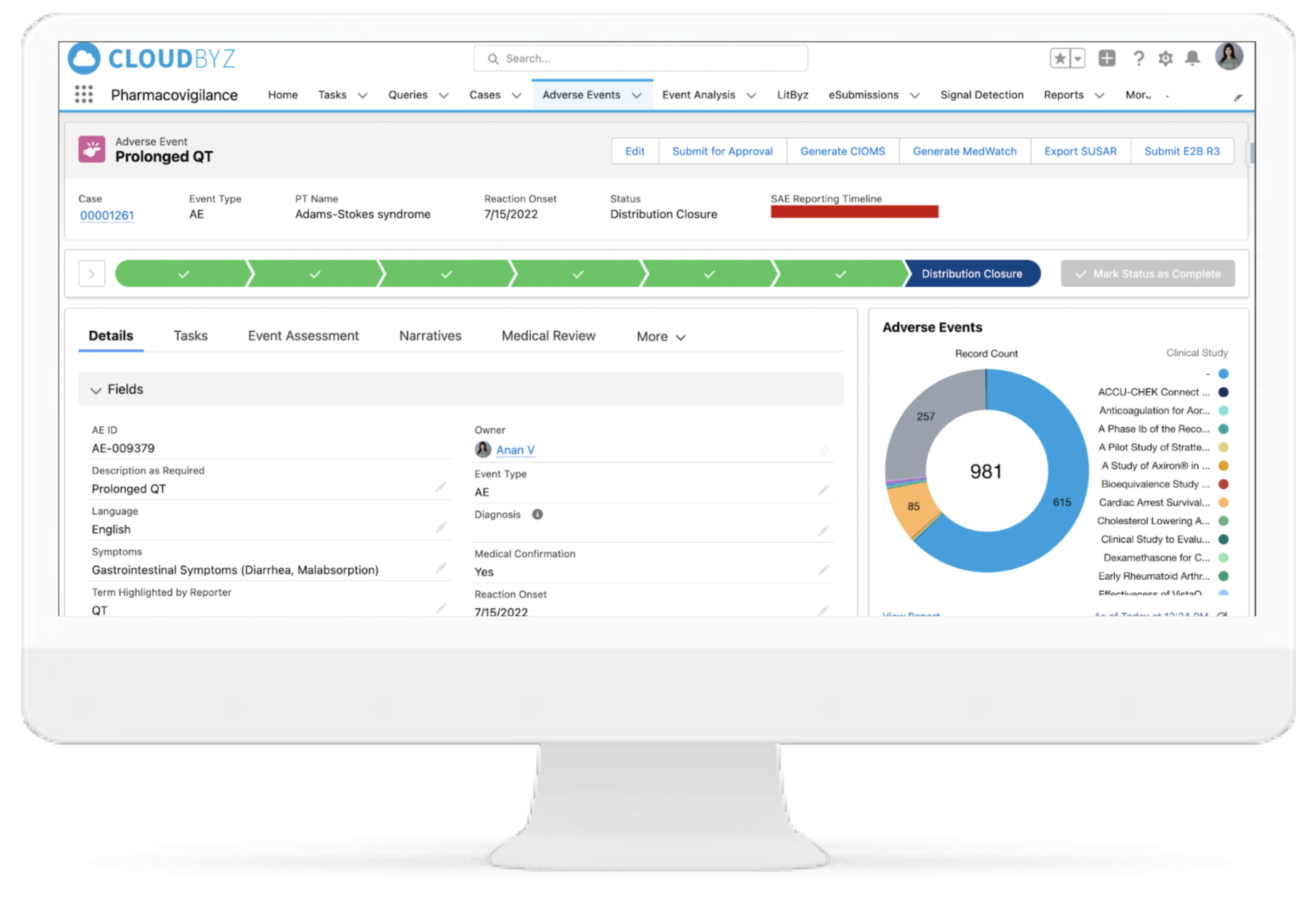
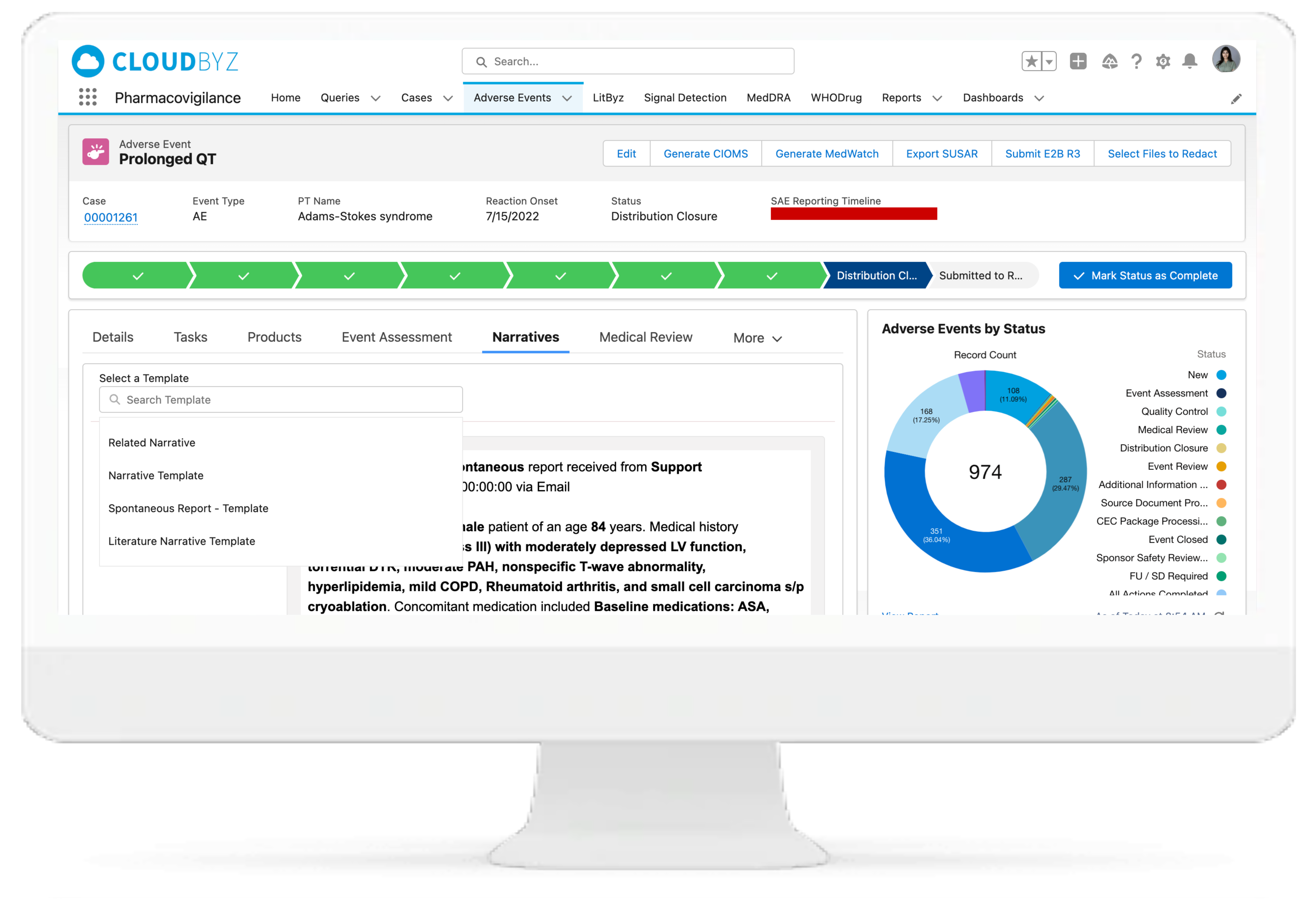
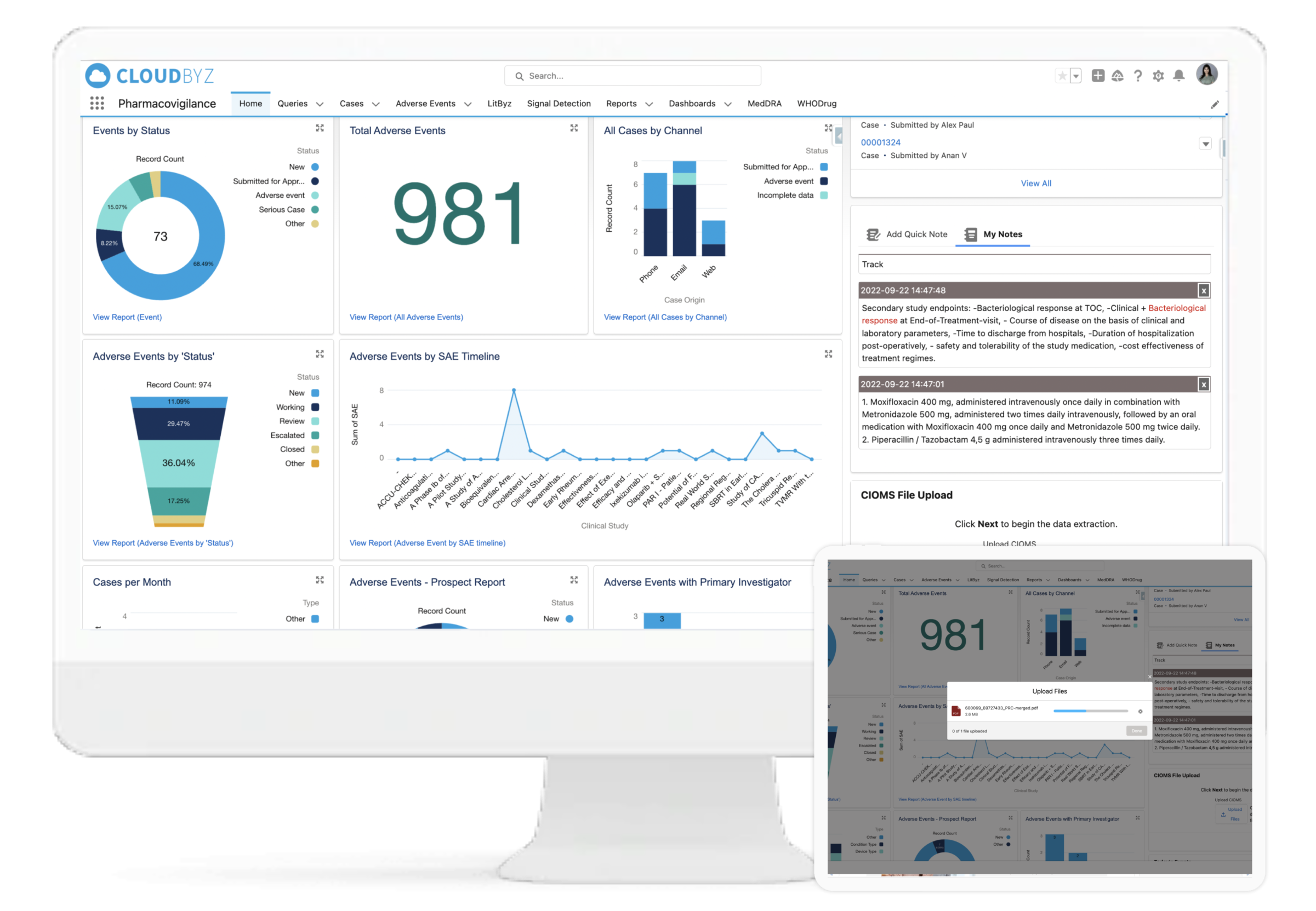
Cloudbyz Safety and Pharmacovigilance solution automates the intake of cases received from various sources and formats (ICH E2B R3/R2) by utilizing cutting-edge technologies. This entire process leads to significant increase in case management efficiency, scalability and enhanced accuracy. Additionally during submission of regulatory reports, users can filter out cases for review, which require submission according to the regulatory timeline.
Configurable Case Workflow Automation
WHO DD/ MedDRA Medical Coding
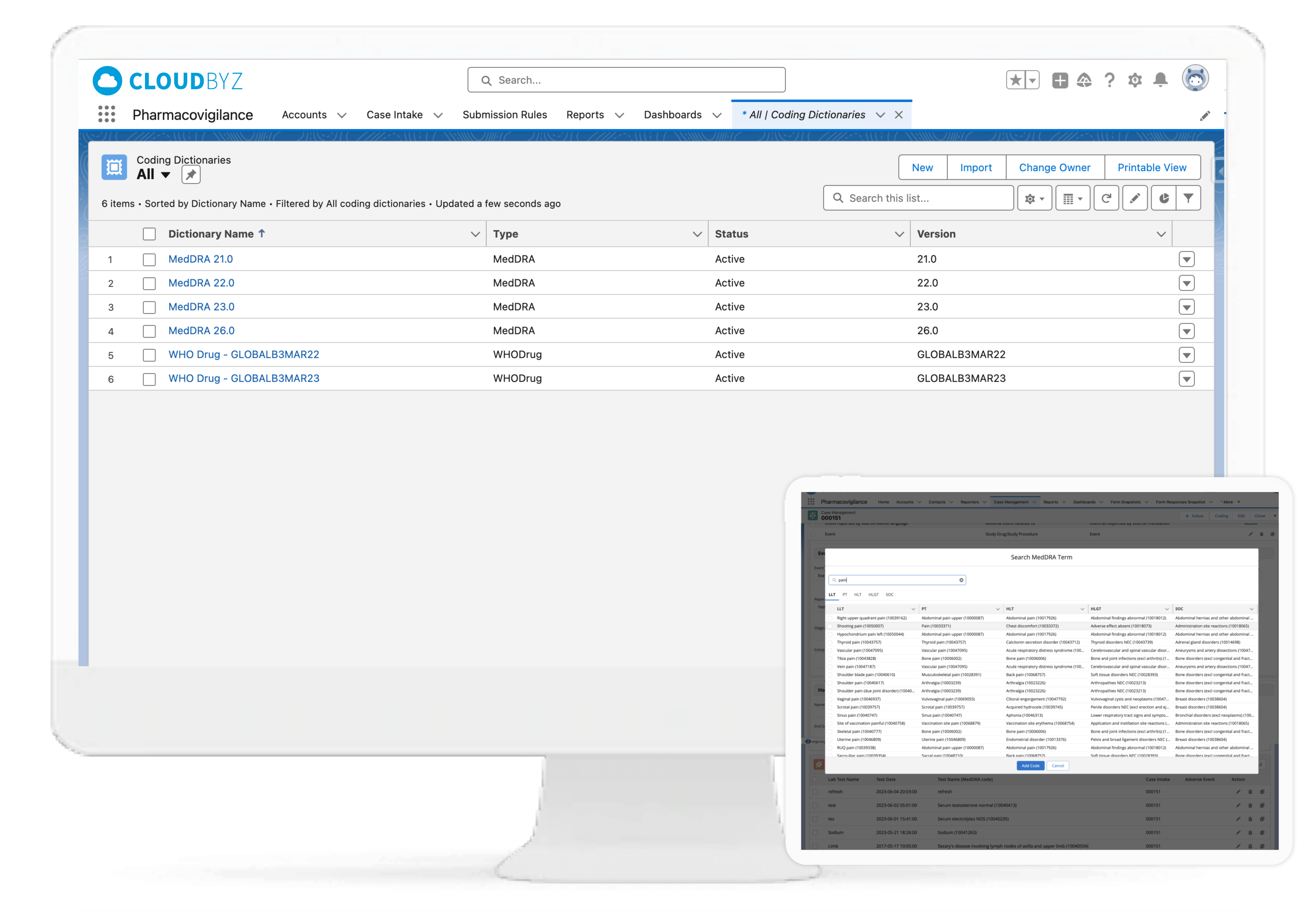
WHO DD/ MedDRA Medical Coding
Leveraging WHODrug and MedDRA dictionary integrations, the Cloudbyz system can enable coding of adverse events, medical histories, laboratory tests, medications, and more as per industry standards. The WHODrug dictionary can be integrated via APIs with the Cloudbyz solution thereby removing any need for upload of the complete dictionary in the system and versioning with every release. Users can version the MedDRA according to each account, project, or clinical study. Additionally, users can select the dictionary type for coding, which can be either library-based or API-based.
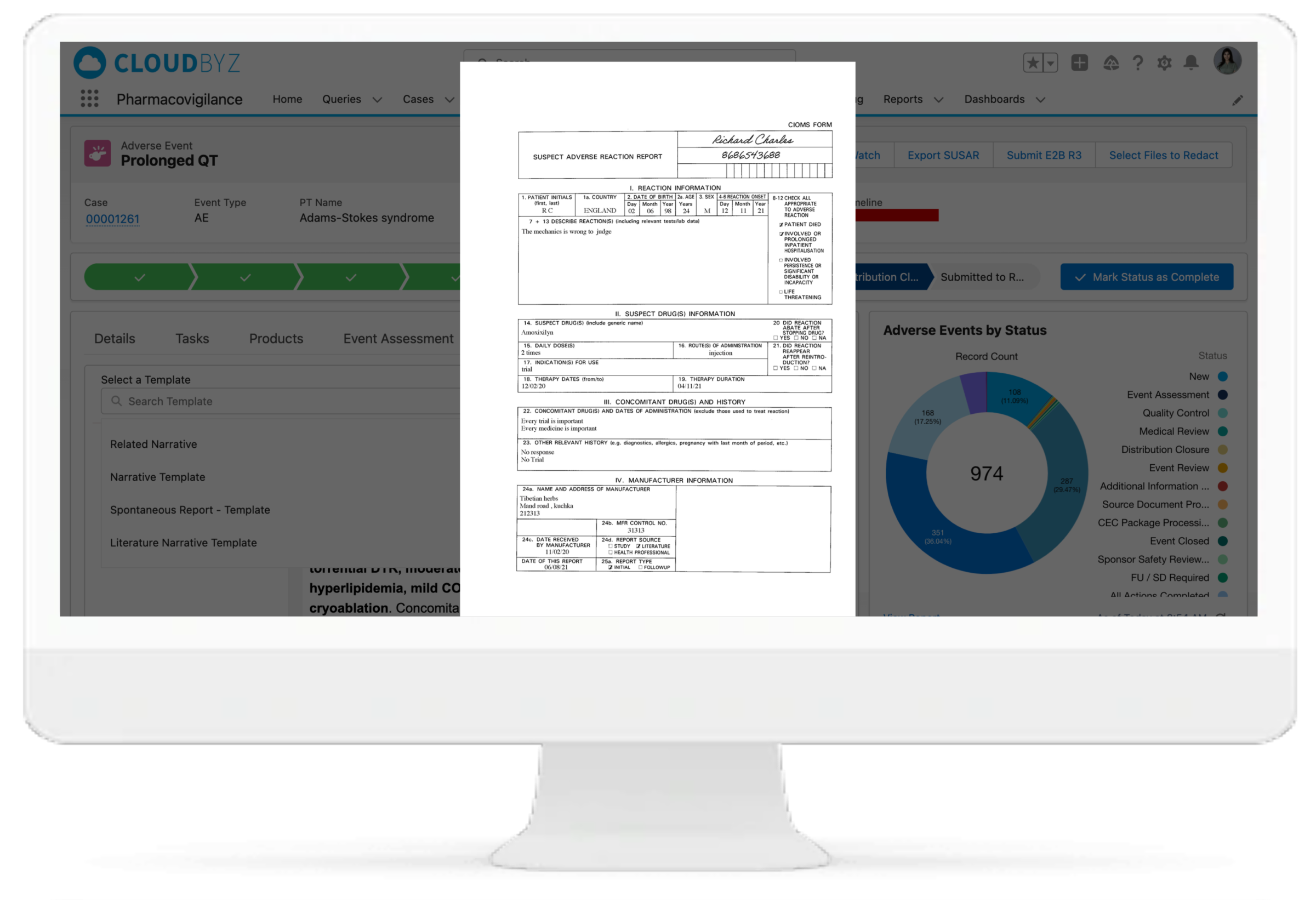
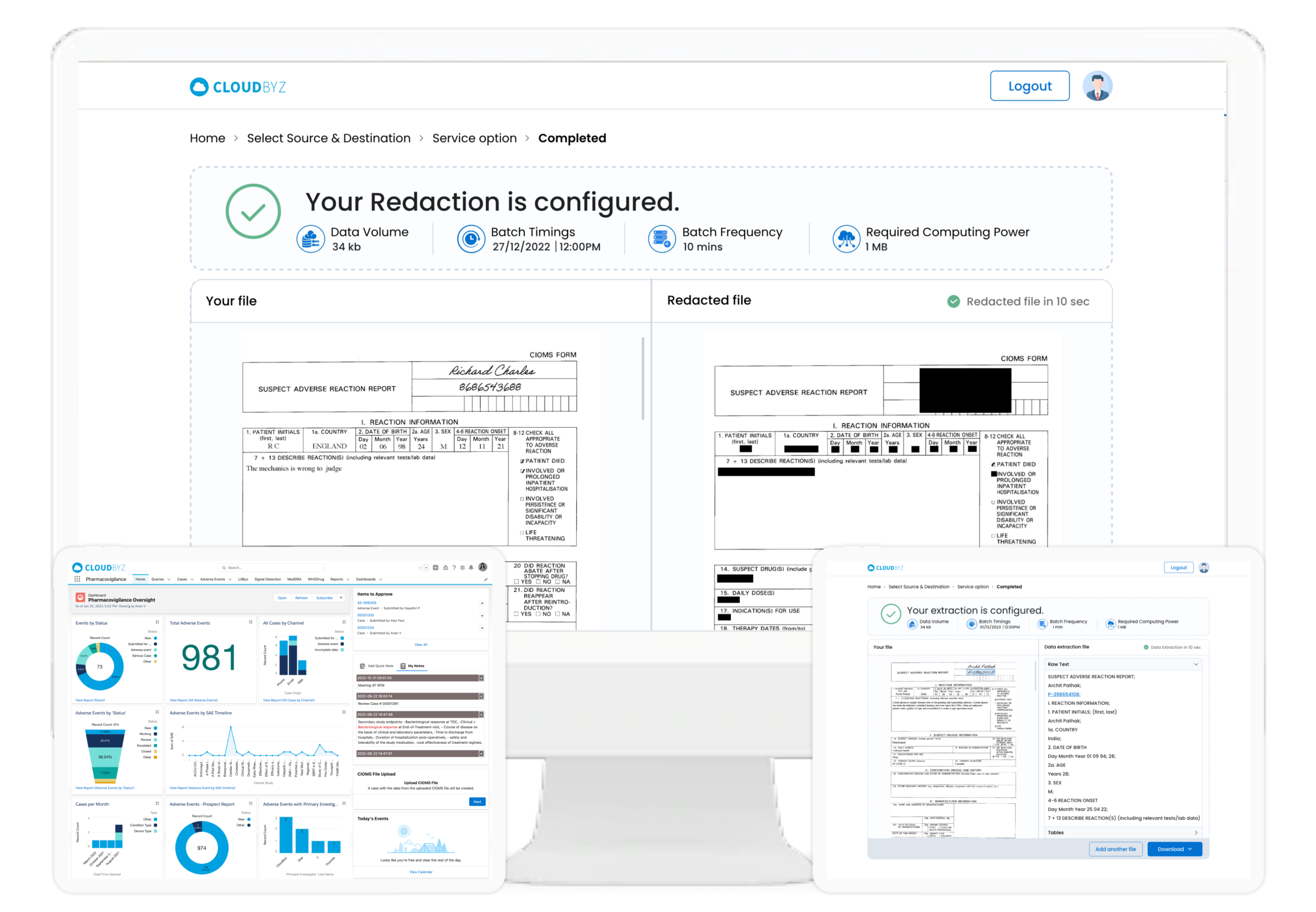
Case Extraction from Unstructured Documents (CIOMS)
Regulatory Submission E2B Gateway
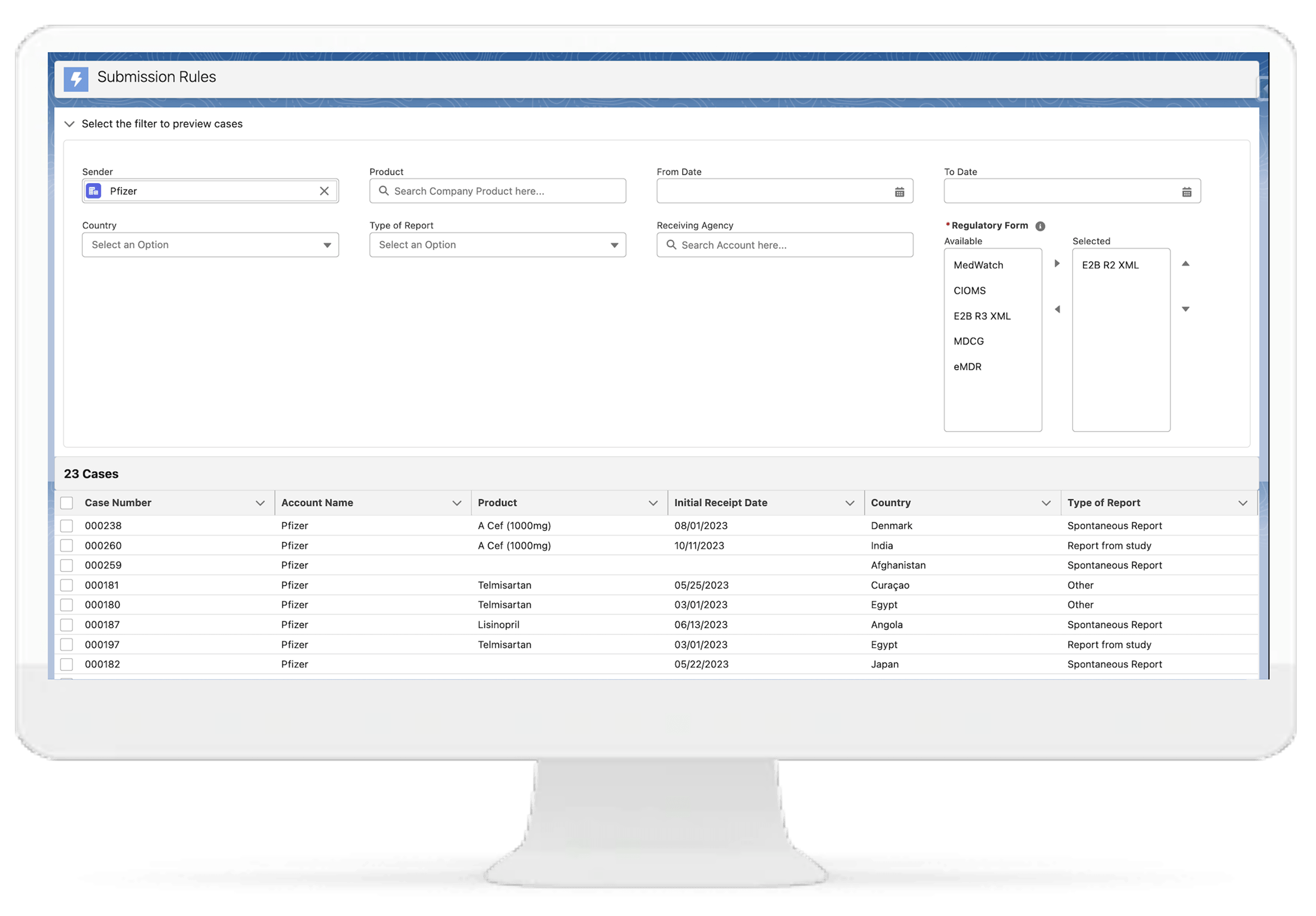
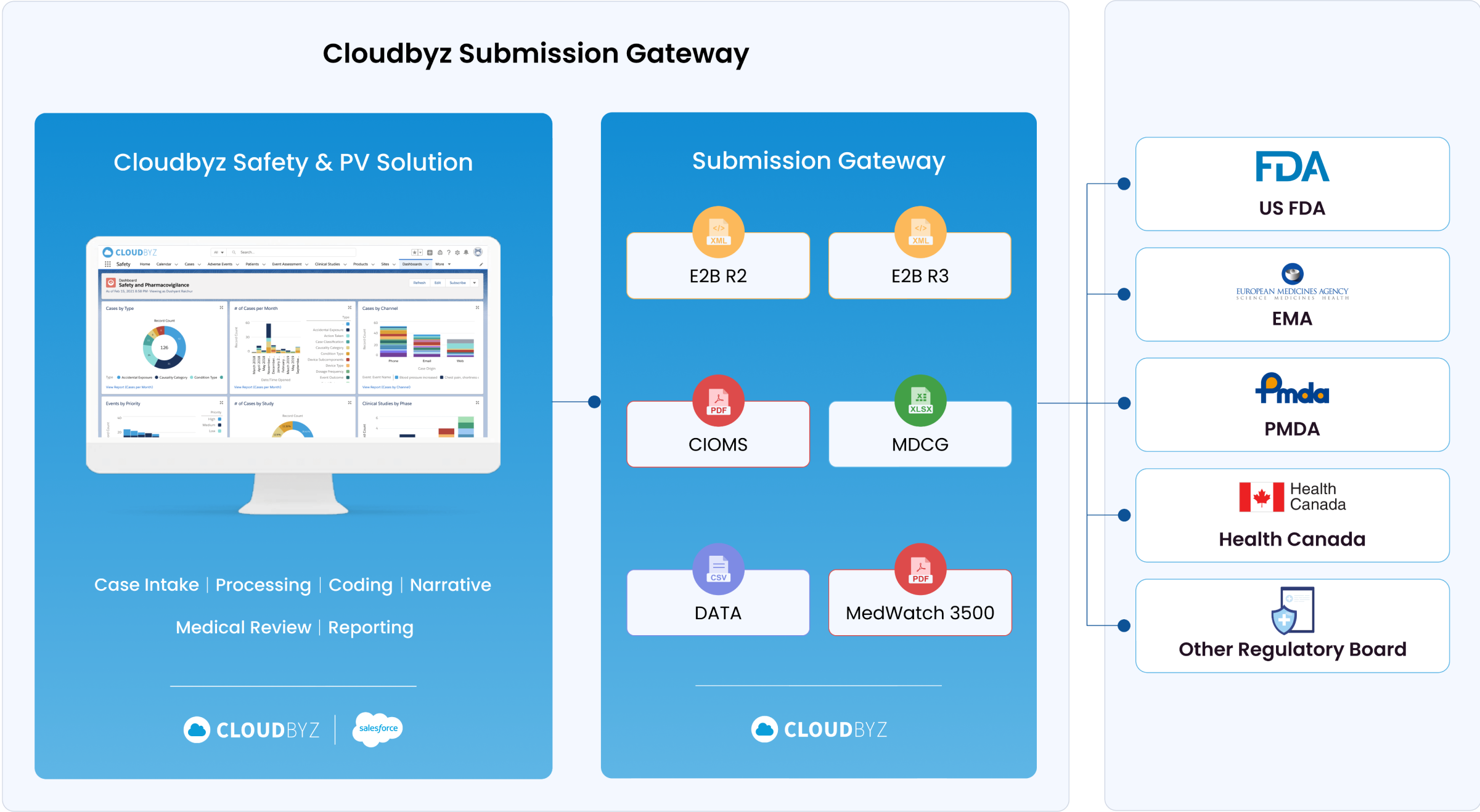
Regulatory Submission E2B Gateway
With our solution, you can seamlessly submit reports to global health authorities in the required E2B R2, E2B R3, MDCG, and MedWatch 3500A formats. This facilitates smooth regulatory interactions and ensures compliance with international pharmacovigilance standards.
Aggregate Reporting
Aggregate Reporting
We help in processing the cumulative safety data from a wide range of sources on a periodic basis and help in regulatory submissions of various reports like PSUR, PADER, PBRER, DSUR, and Ad-hoc reports. Our system is capable of generating case line listings at MedDRA level for individual products with auto generated templates designed as per client SOP.
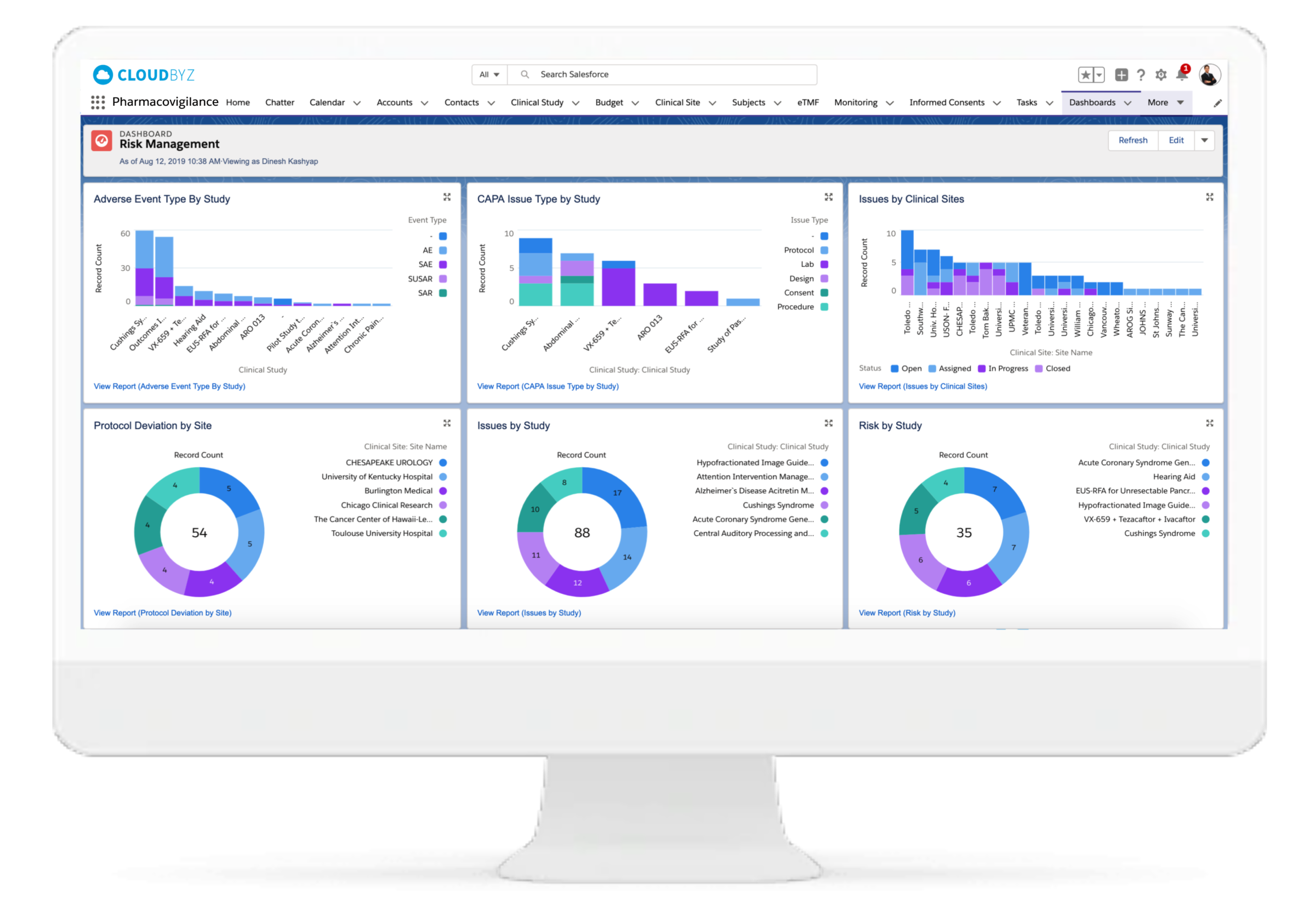
Unified with Cloudbyz Clinical Trials Management
Signal Management
Signal Management
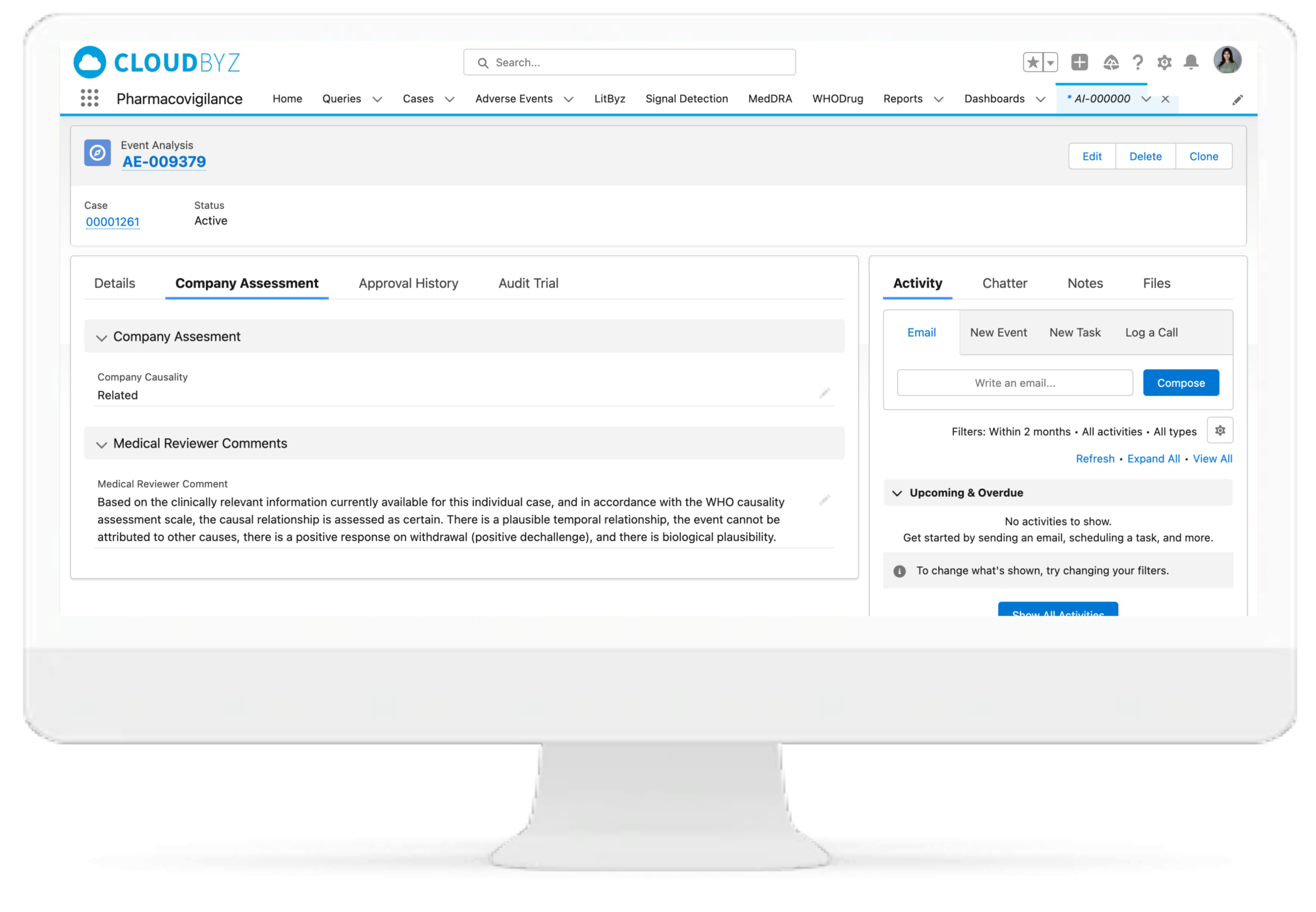
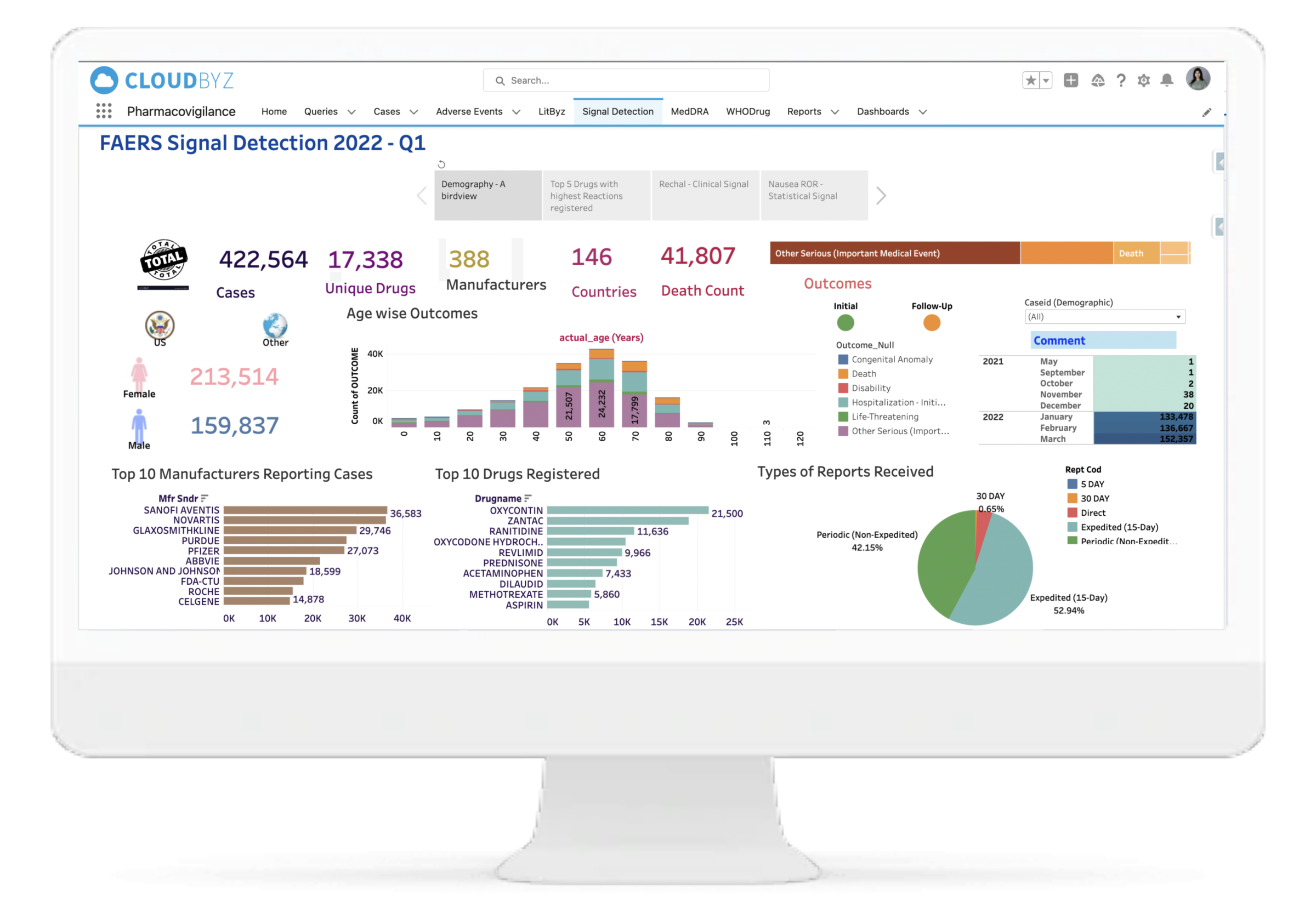
Our system assists medical experts in making decisions, saving you valuable time and requiring less effort to go through each and every case for signal detection. Our signal management system helps in signal detection using well defined statistical approaches with a set Signal Disproportionate Report threshold, signal validation using predefined customizable algorithms for case by case analysis, and signal prioritization based on defined criteria to help the clients with signal assessment. We also help in FAERS and EVDAS reviews for products by monitoring SDR and ROR as defined. With Litbyz, we also help in gathering additional supportive data for signal assessment.
Benefits
By opting for Cloudbyz's Pharmacovigilance solution, you stand to gain: