TRUSTED BY
Cloudbyz EDC is a user-friendly, cloud-based solution that is designed to capture and manage clinical data effectively throughout a clinical trial’s life cycle. Our innovative solution enables clinical research teams to efficiently collect, analyze, and manage clinical data of different complexity and size.
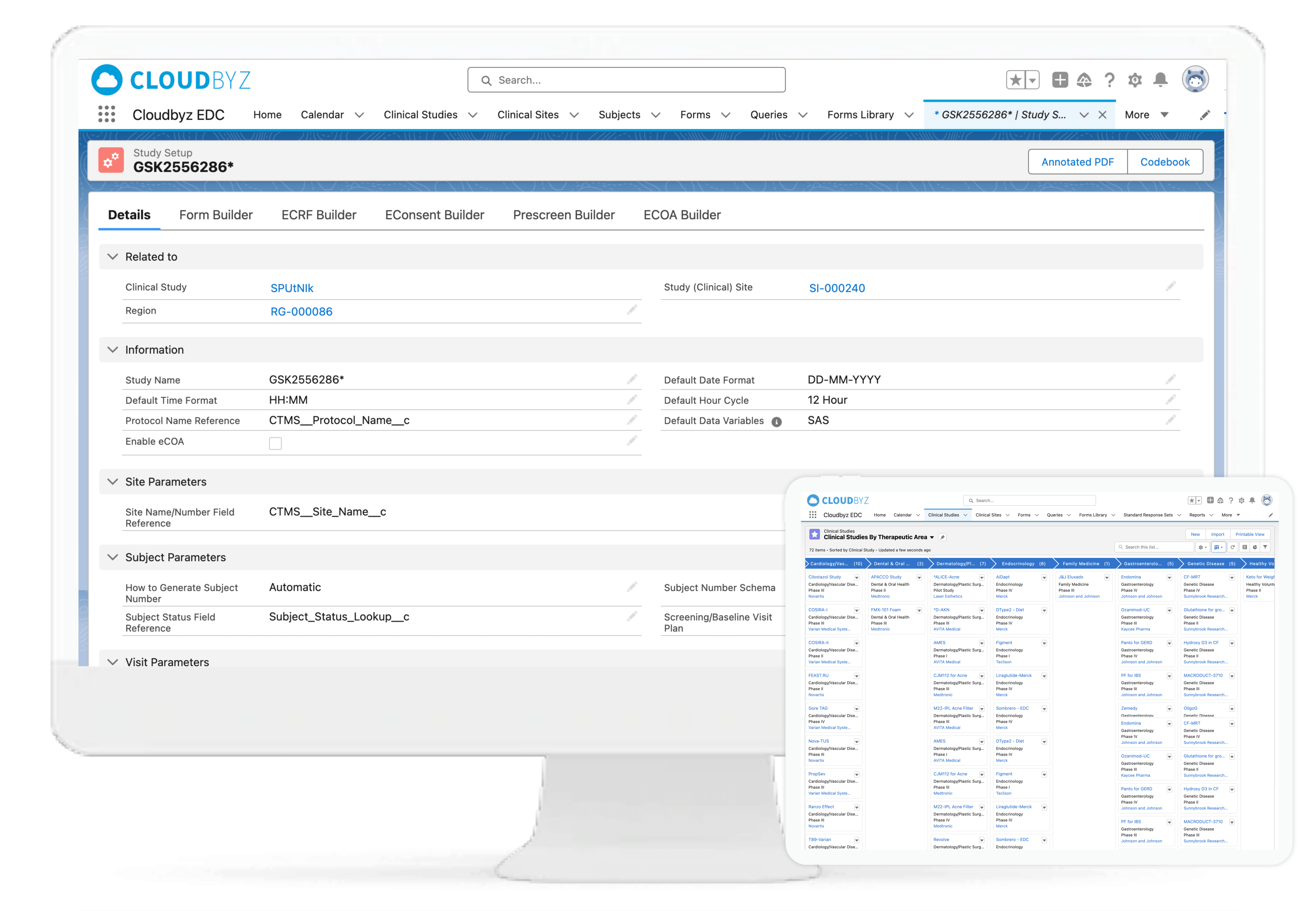
Cloudbyz EDC enables rapid study build with point and click with an interface that helps users build forms and easily navigate to the appropriate screens for data collection and analysis.





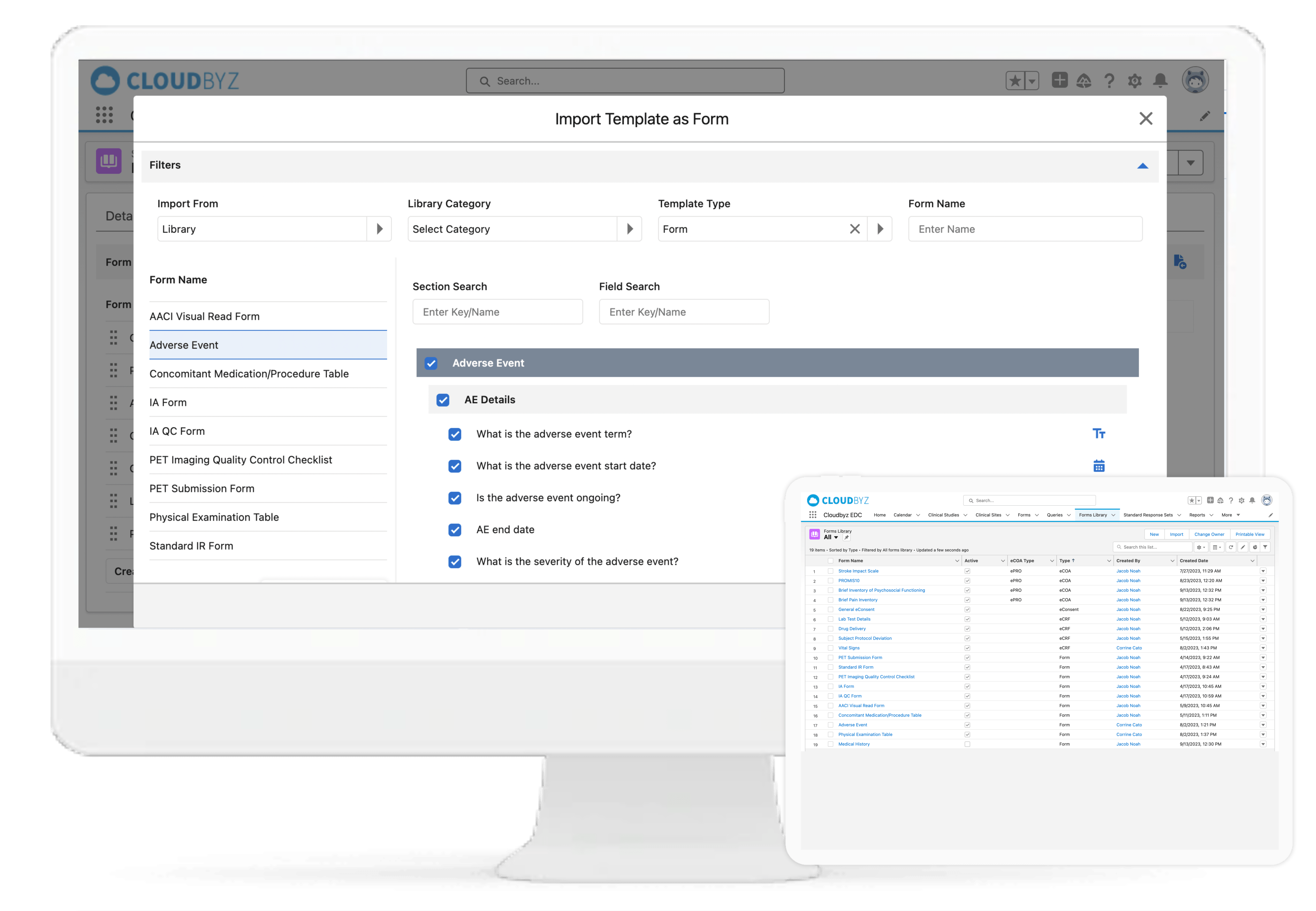
With Cloudbyz EDC solution, go live with your studies faster and with ease by creating simple to complex forms and reusable form libraries. A cloud-based, easy-to-use solution that enables rapid study build, efficient data collection, data monitoring and data cleansing. Decrease efforts and costs involved in the data management process.

Our robust rules engine allows you to create complex forms and set up multiple validation rules at the field level.
Define data validation checks and validation messages, using Cloudbyz Rules Engine. Cloudbyz rule builder will enable users to define the expression that will be executed to be true for data validation checks. Assign actions such as stopping the user from entering the wrong data but making the system raise queries and send notifications to the appropriate roles in the case of soft validations





Cloudbyz eConsent replaces the paper-based informed consent document with interactive, multimedia enabled, and template-driven informed consent on mobile devices. The solution is fully integrated with Cloudbyz EDC enabling real time visibility and central tracking of enrollment metrics across the sites.

Cloudbyz Randomization solution is flexible and scalable to meet the unique requirements of each study. Effortlessly handle simple to complex randomization schemes with provision for stratifications and multi arm studies. Cloudbyz Randomization module supports and uses its own algorithms for all the randomization types such as Simple Randomization, Block Randomization, Permuted Block Randomization (Stratified Block Randomization)
Comes pre-integrated with the EDC, Cloudbyz Randomization module helps studies maintain balance between treatment groups among subjects they recruit, it is easy for statisticians to generate the randomization schedule by just defining the parameters from a schedule generator form. The schedule is generated automatically without requiring any coding.





Cloudbyz eCRF (electronic case report form) is a cloud-based, easy-to-use solution that enables rapid data collection, more efficient data cleaning and an overall decrease in the efforts and costs involved in the data management process. Our solution, built on the Salesforce platform, is 21 CFR part 11 compliant and has features such as data back-up, multi-language abilities, security features such as access control and data encryption, multimedia content support and customizability.

Cloudbyz eCOA solution leverages innovative technologies to capture the clinical-outcome data through easy to use interfaces. This includes ePROs, clinician reported outcomes and eDiary functionalities. Our solution is device friendly, supports time-sensitive data collection, enables upload of multimedia content for better patient education, and uses various edit checks to minimize data errors. Set up patient reminders and notifications to collect close to 100% data from patients.


