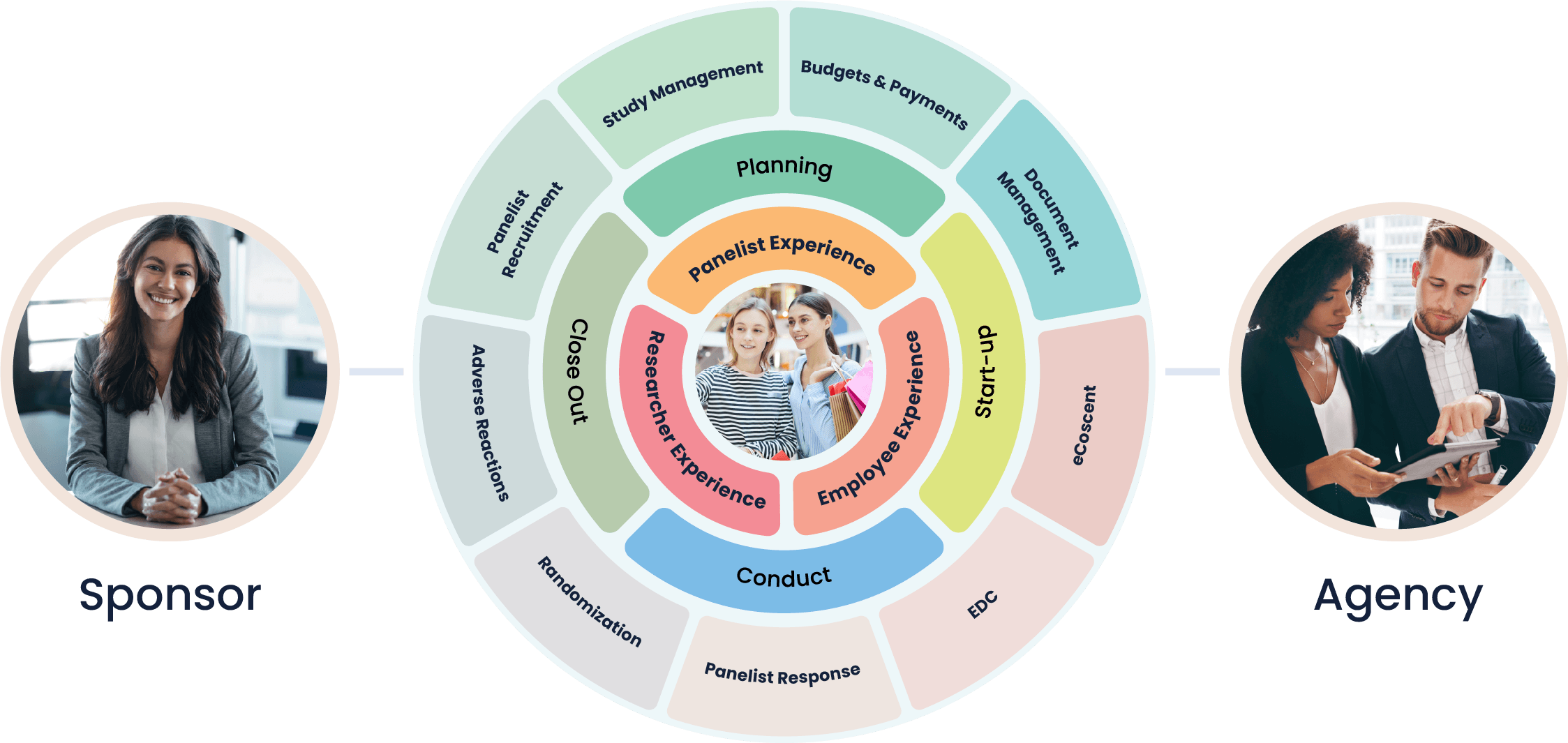
At Cloudbyz, we understand the unique challenges and requirements of the specialty chemicals industry. We provide tailored, unified clinical trial management solutions designed to streamline operations, enhance safety and compliance, and deliver actionable insights to drive innovation and competitiveness.