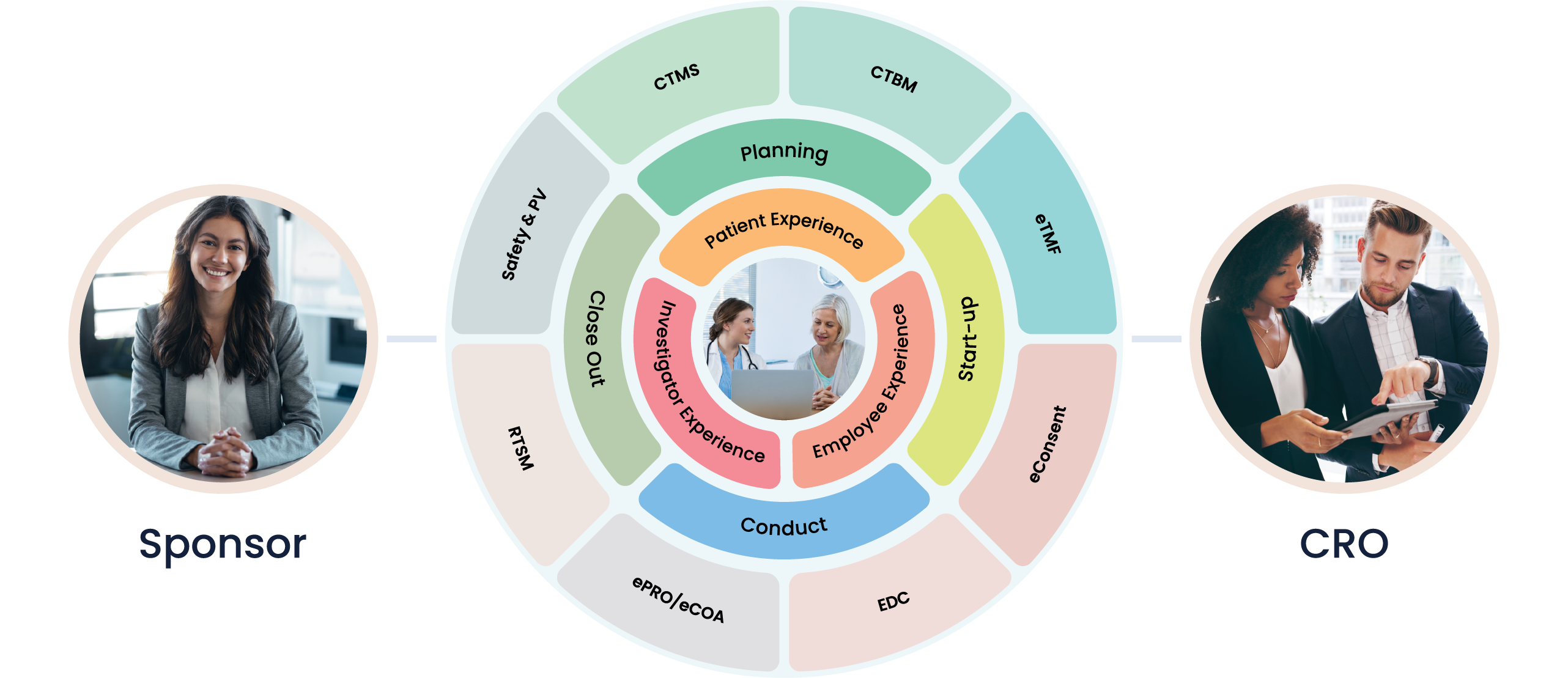
At Cloudbyz, we understand that Site Management Organizations (SMOs) play a crucial role in the clinical trials landscape. Ensuring that sites are optimally managed, properly resourced, and fully compliant is a significant task. That’s why we’ve developed a unified clinical trial management solution specifically tailored to the needs of SMOs.