TRUSTED BY
Digital Clinical Research Platform For Sites
Built on the innovative Salesforce platform, Cloudbyz’s unified clinical research management solutions for Clinical Sites, Site Networks, Site Management Organizations (SMO) and Academic Research Networks (ARNs) enables seamless collaboration across clinical trials processes and workflow between teams and key stakeholders including sponsors, CRO, IRB and Patients.
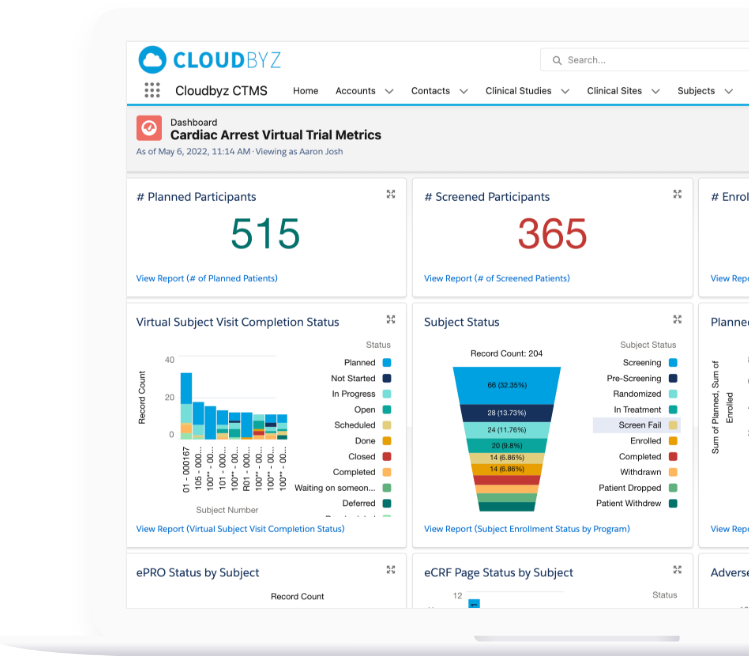
The platform enables patient recruitment, trial management, electronic data capture, remote monitoring, regulatory documentation management, real-time collaboration with patients, sponsors, CRO and enables integration with medical devices for direct data capture.
Cloudbyz unified clinical research platform comes with integrated capabilities as one system and the solution is secure and compliant with 21 CFR Part 11, ICH-GCP, GDPR, HIPAA and other global regulatory requirements. Cloudbyz platform is configurable, flexible, scalable and easy to use.
- 65% improvement in productivity.
- 50% increase in study intake with same staff
- 90% eliminated paper based processes
- 80% improvement in Quality
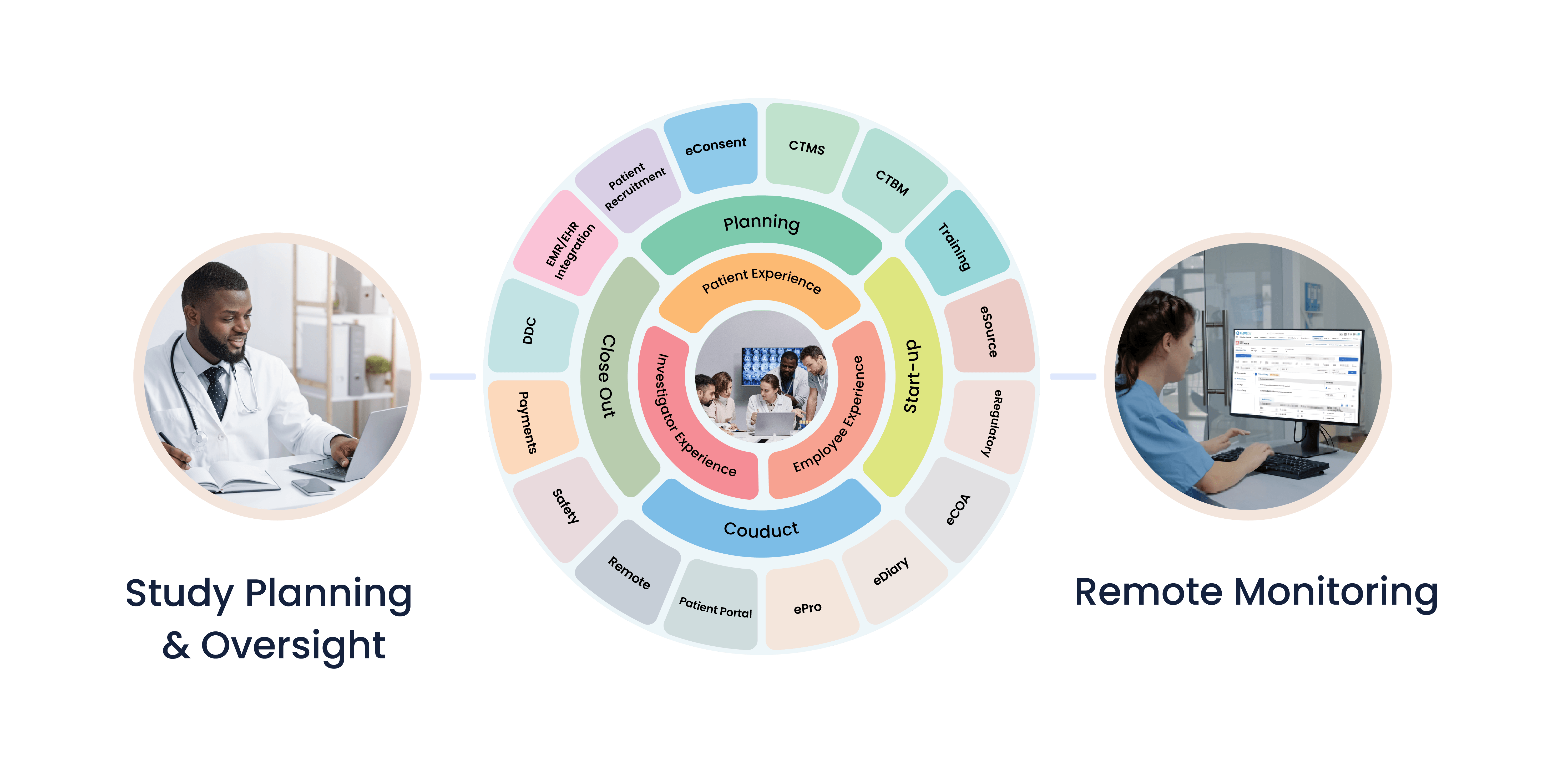
Product Features
Patient Recruitment
Patient Recruitment
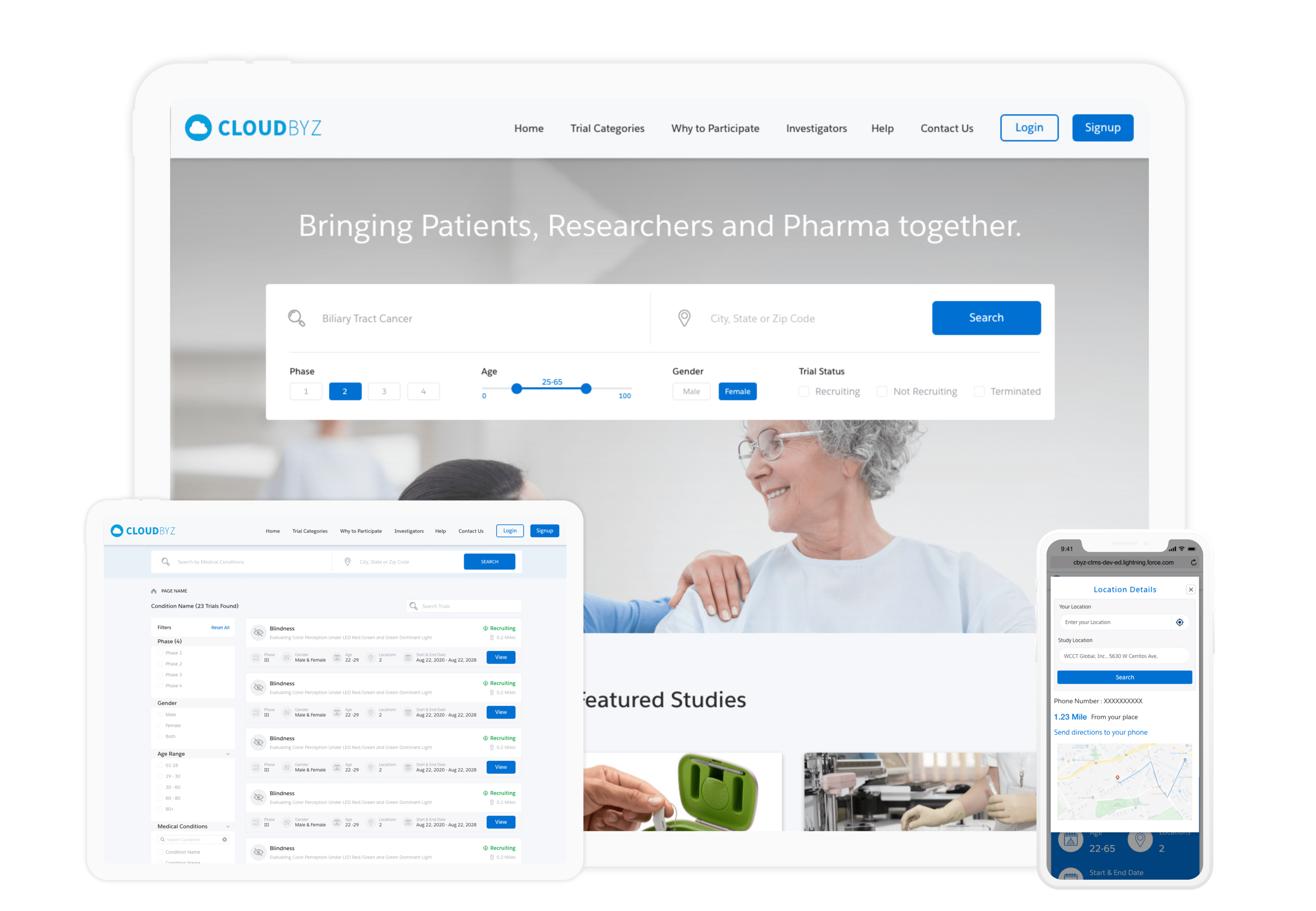
Cloudbyz Patient Recruitment offers complete end-to-end patient recruitment SaaS support across all types of clinical trials. The solution empowers sponsors, CROs, and sites to digitize the entire recruitment process along with smart features such as automatic roll-ups, automated patient-study matching, better metrics, and improved analytical capability.
Effectively engage patients to drive better patient experience by simplifying the entire recruitment process from first contact to prescreening and through to eConsent and screening. Learn more about our Patient Recruitment solution here.
CTMS
CTMS
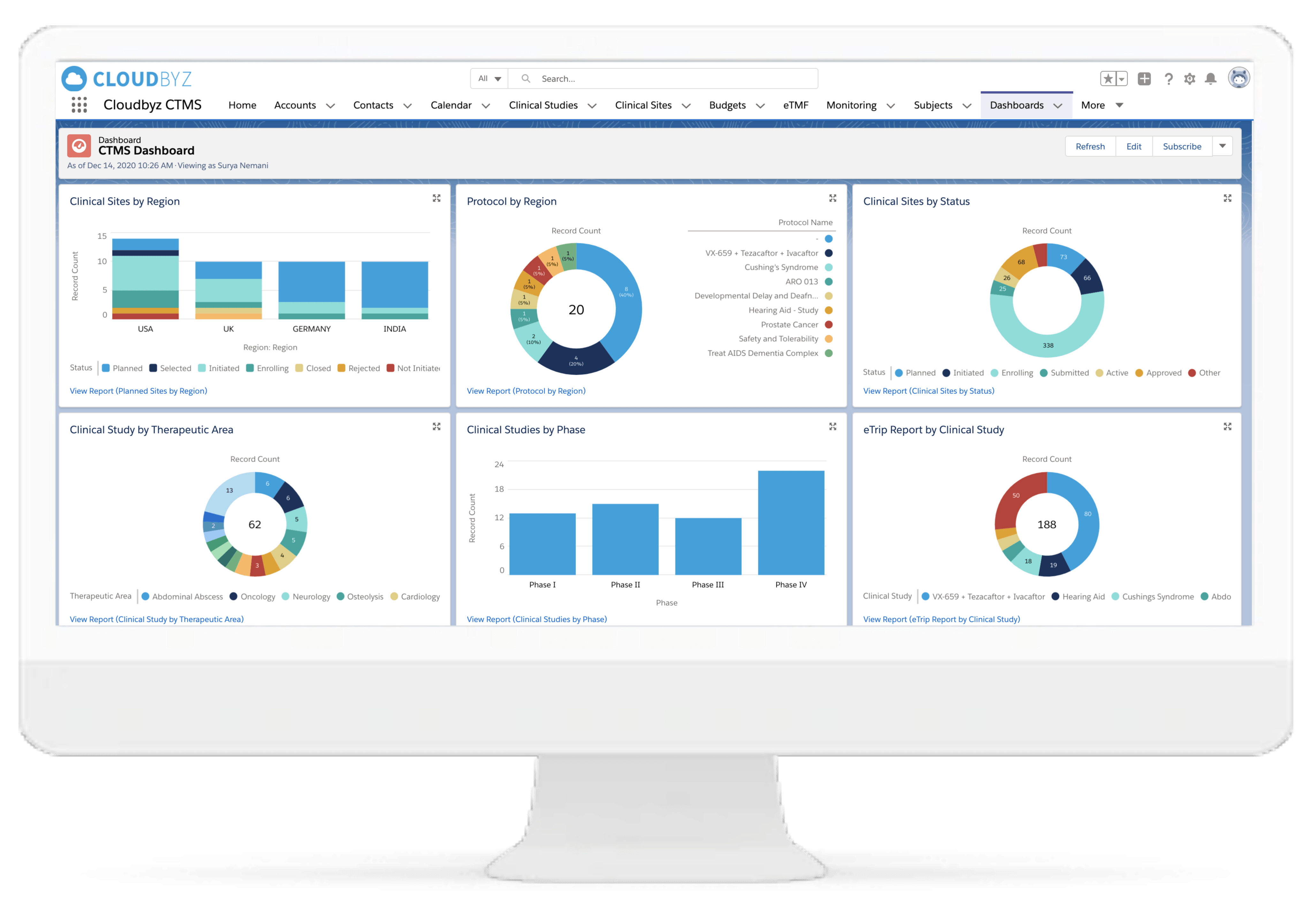
Cloudbyz Clinical Trial Management System (CTMS),built on the Salesforce cloud platform, is a unified, integrated clinical trial operations management solution. It offers end-to-end management of clinical trial operations with real-time visibility and analytics across study planning, budgeting, study start up, study management, and close out. Learn more about our CTMS here.
eSource
eSource
Cloudbyz eSource is a user-friendly, cloud-based solution designed to capture clinical source data directly in an electronic format, enabling research sites to effectively collect, manage, retrieve and recycle data throughout a clinical trial’s life cycle. Learn more about the Cloudbyz eSource here.
eRegulatory
eRegulatory
Cloudbyz eRegulatory offers a cloud-based repository of all clinical trial documents. Digitally capture, manage, share, and store all clinical trial-related documents with a centralized overview. Stay inspection ready by managing essential trial documents and leveraging automations while enabling real-time visibility for CROs, sponsors, monitors, and other stakeholders. Learn more about the Cloudbyz eRegulatory here.
Payments
Payments
Generate payments, create detailed invoices, view payment tracking history, and manage study and site level budgets with the Cloudbyz CTBM solution. Leverage template-based modules to set up multiple budget versions at the study and site level. Simplify accounting practices by managing and tracking advance payments to sites, partial payments, unscheduled and invoiceable items payments and holdback payments. Designed to fit your financial needs, set up different payment frequencies per site such as monthly or quarterly payments along with robust approval workflows to keep your business on track.