TRUSTED BY
Real World Evidence (RWE) gathers data from diverse sources like EHRs, wearables, and social media. Unlike clinical trials, it shows how treatments work in real-world scenarios, offering insights into their impact on various patients over time.











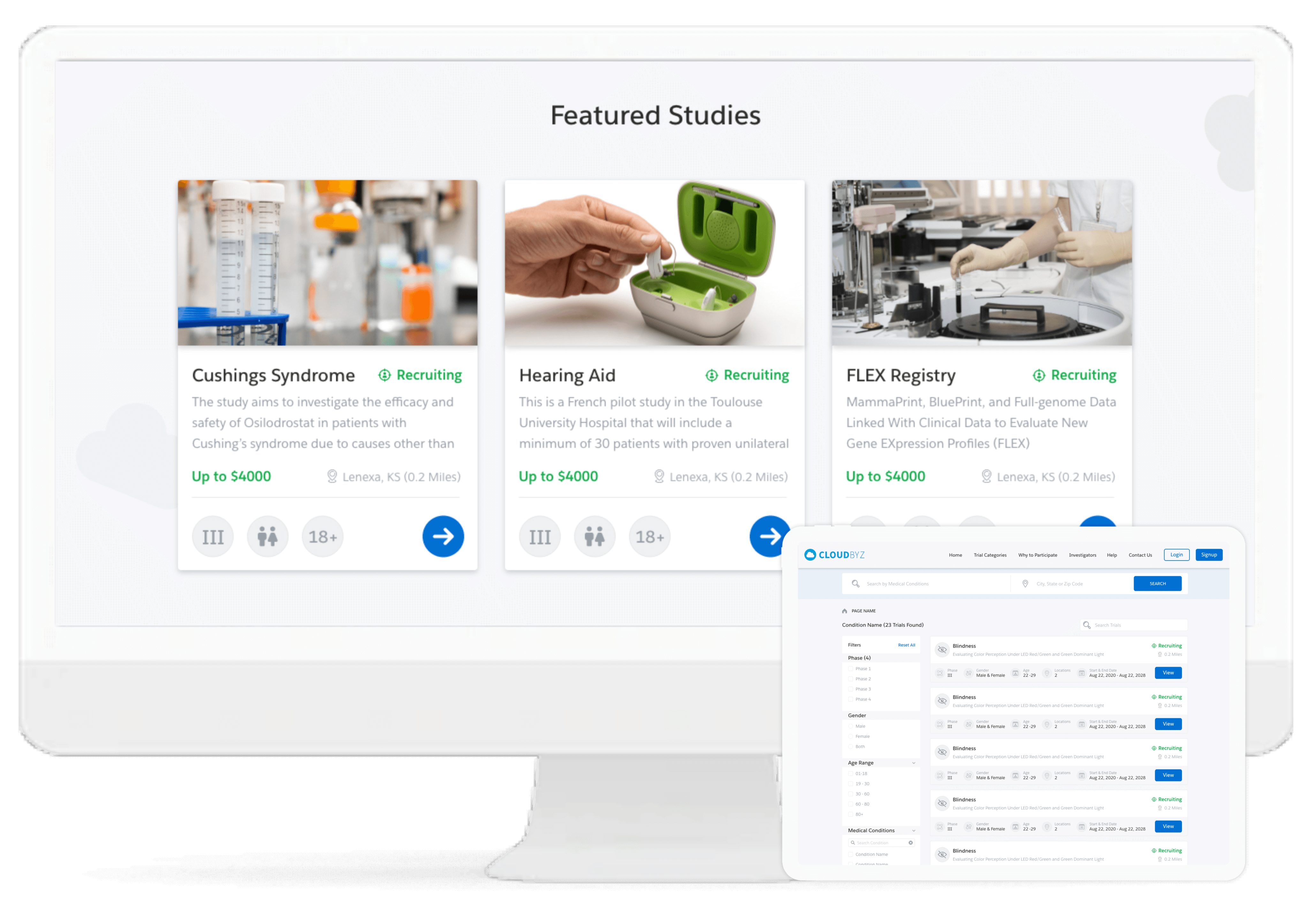
Cloudbyz investigator recruitment, a pioneer in this functionality, enables investigators to gain easy access to feasibility study of any existing/potential site with regards to clinical trials. We facilitate mass email distribution (using the investigator database) targeted to different investigators with accessible site feasibility links.





Cloudbyz enables comprehensive study, site and end-to-end project management of the clinical trials through its different CTMS modules. It delivers complete off-site and hybrid-based process streamlining including site feasibility study, report monitoring, study management, IRB approval tracking, training, real-time performance tracking, among others.





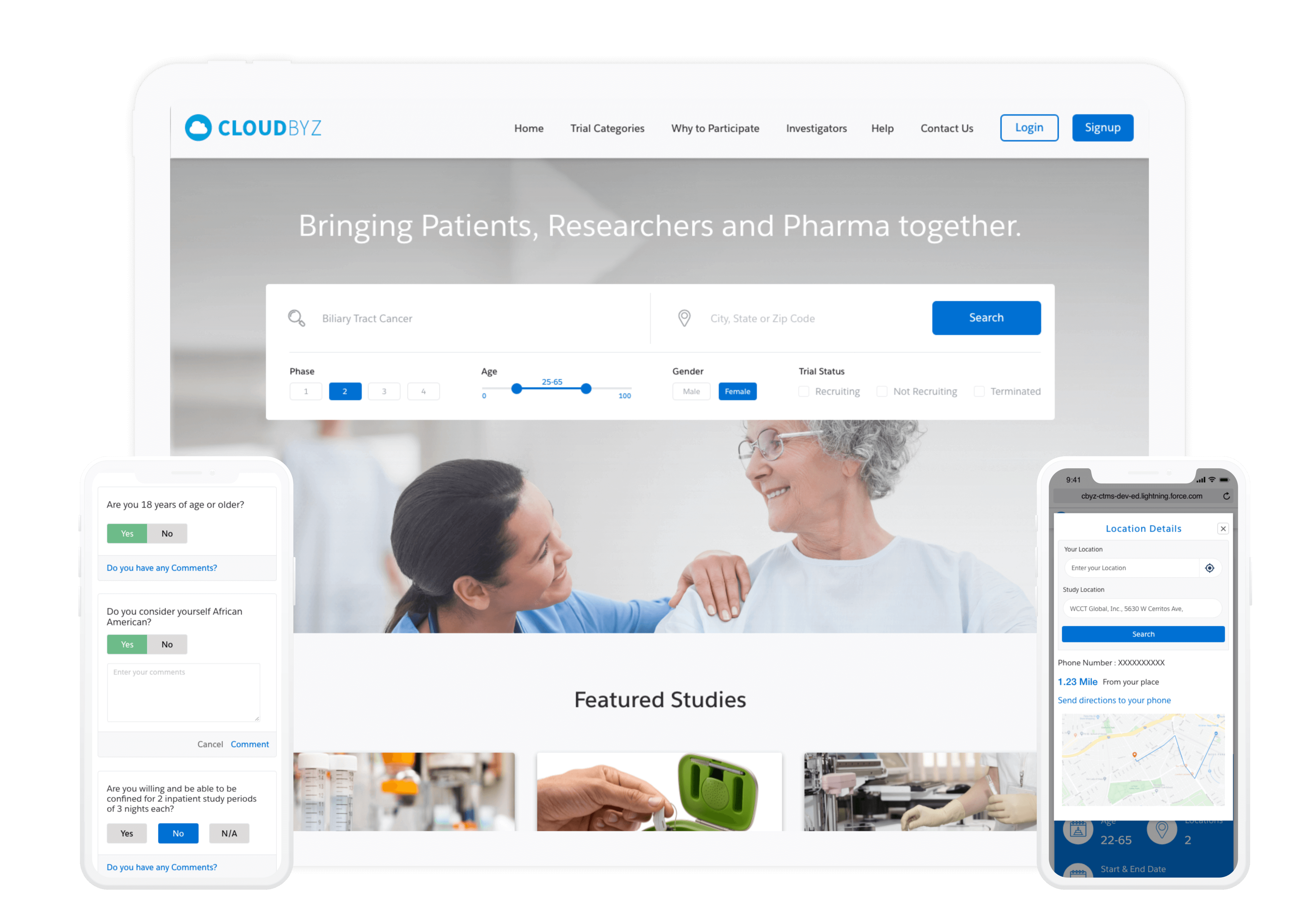
Cloudbyz supports virtual visits for investigators/CRO and patients by generating visit appointments as per the schedules and maintaining schedule calendar. Telemedicine functionality is used for routine follow-ups to identify adverse events. It also helps in setting up procedures, subject visits and payment policies depending upon the budgets and study protocols.





Cloudbyz RWE budget solutions enable a hawk-eye budget estimation, planning, payments management across clinical trials. Cloudbyz delivers defined budget templates, real-time tracking of investigator grants, procedures and visit payments along with budget projections and variance between planned and realized expenses.










Cloudbyz solution offers electronic data capture (EDC), with product features for source data validation (SDV), questionnaire templates, queries and eSignature and transcribes them into systems. It facilitates faster data processing and centralized maintenance of clinical patient data.





Cloudbyz Real World Evidence solution enables capturing patient data electronically both from wearables and manually entered information by investigators. Patient health data (like heart beat, SpO2,etc) from multiple gadgets, on a real-time basis are extracted through integrations. Also other data like medical history can be updated and rolled-up post manual feed on the participant portal.










Cloudbyz recognizes the integral role of pharmacovigilance in maintaining drug safety and regulatory compliance in the healthcare industry. We've designed a cutting-edge solution that tackles the unique challenges of this domain, simplifying processes, enhancing efficiency, and offering reliable, actionable insights.







Real World Evidence (RWE) gathers data from diverse sources like EHRs, wearables, and social media. Unlike clinical trials, it shows how treatments work in real-world scenarios, offering insights into their impact on various patients over time.

Our solution consolidates data from diverse sources, providing a comprehensive understanding of patient health, outcomes, and interventions.

Advanced analytics and reporting capabilities enable organizations to derive valuable insights and drive evidence-based decision-making.

Conduct patient-centric outcomes research to evaluate interventions, identify best practices, and enhance patient care.

Swiftly identify safety concerns, manage adverse events, and monitor patient outcomes in real time.

Foster collaboration and secure data sharing among healthcare stakeholders to drive research, innovation, and improved patient outcomes.

Built on the Salesforce platform, our solution offers scalability, reliability, and robust security measures to protect patient data.
Ready to join the Cloudbyz CRO Partnership Program? Contact us today to learn more about how we can work together to advance your clinical trial management process.